Does Potassium Chloride Have a High Melting Point
Why does Magnesium Oxide have a higher melting point than Sodium Chloride. Stronger interactions mean you need more energy to separate the molecules.

Vanadium Boride Vb2 Powder Funcmater Ceramic Materials Tungsten Tungsten Carbide
What is Potassium Chloride.

. 9Potassium chloride Plus 1 point. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. The melting and boiling points of potassium chloride are 1040 K and 1690 K respectively.
Can be from 4-D sources. Potassium chloride CAS Number. Its melting point is about 770 C and the boiling point is 1420 C.
All ionic compounds have high melting points for this reason. Note that these points are associated with the standard atmospheric pressure. Why does calcium fluoride have a high melting point show 10 more the loop of henle.
Suggest foods containing high potassium. 198 gmL at 25 Clit Boiling Point. As the ionic lattice contains such a large number of ions a lot of energy is needed to overcome this ionic bonding so ionic compounds have high melting and boiling points.
However a mixture of LiCl 55 and KCl 45 melts at about 430C and so much less energy and so expense is required for the electrolysis. Sodium chloride is made from Na ions and Cl-ions and has a melting point of 801C. Answer 1 of 3.
Why does potassium chloride have a high melting point. Ionic compounds are held together by electrostatic forces between the oppositely charged ions. Since more energy is required to break the bonds the melting point of calcium will be higher than that of potassium.
Both Magnesium Oxide and Sodium Chloride exist as a giant ionic lattices where each oppositely charged ion is held in place by a strong electrostatic attractions. The substance with the highest melting point is going to be the one that has the strongest inter molecular forces. The electrostatic force between the positive ions cations and the delocalized electrons keeps the structure intact.
Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions. It is because the distance between the calcium and chloride ions nuclei is less than the distance between the mercury and the chloride ions nuclei given the fact that mercury atom has more number of orbitals and shells than calcium. Does KCL have a high melting point.
What is the melting point of LICL. Why does strontium chloride have a high melting point. Both potassium and calcium are metals.
If all we have are molecules. Coloring agents should never be used in Pet Food. Ionic substances all have high melting and boiling points.
Does potassium chloride have a high or low melting point. These ionic bonds are strong and require a large amount of thermal energy to overcome them and break the. Magnesium oxide is made from ions with two charges Mg 2 and O 2- and so has a much higher melting point of.
Covalent bonds are very strong look at diamond the boiling point is very high. Why does LiCl have a higher melting point than ccl4. Which chloride has the highest melting point.
This requires more heat energy to overcome. High 772 degrees Fahrenheit Does potassium have a high melting point. So lithium chloride will have the highest melting point while all the others are molecules or an.
The difference in melting points for ionic compounds can be explained by the size of the ions themselves. Potassium chloride or KCl is an ionic solid. Standard source of potassium balances acidalkaline levels.
Does ch4 have a high melting point. Sodium chloride has a high melting and boiling point There are strong electrostatic attractions between the positive and negative ions and it takes a lot of heat energy to overcome them. Potassium chloride is mainly useful in making fertilizers since plants need potassium for their growth and development.
Calcium atoms have smaller radii than potassium atoms since calcium atoms. Potassium chloride KCl or ClK CID 4873 - structure chemical names physical and chemical properties classification patents literature biological activities. 10Bone phosphate Minus 1 point.
Potassium chloride is highly soluble in alcohols but not soluble in ether organic compounds with the formula R-O-R. Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. Non-digestible source of calcium can lead to digestive upset.
At 0 o C 20 o C and 100 o C the solubility of KCl in water corresponds to 2171 gL 2539 gL and 3605 gL respectively. Example help RedoxIGCSE Testing for Ions Confusion aldehyde. Thus their bonding is metallic where the atoms form a lattice shape and share their valence electrons throughout the structure.
It is in the form of white colour. Explaining melting points It takes a lot of energy to overcome the strong electrostatic forces of attraction between oppositely charged ions so ionic compounds have high melting and boiling points. Kidneys Why does Hydrogen Iodide have a higher melting point than Iodine Chloride.
High 772 degrees Fahrenheit. Melting point and boiling point is determined based on how neighboring molecules interact with each other. And since temperature is a measure of kinetic energy this makes sense why higher temperatures th.

The Dangers Of Potassium Chloride Ice Melt Safe Paw

What Is The Melting Point Of Nacl Sodium Chloride So High Youtube

Binary Phase Diagram Of Kcl And Nacl Liq Is A Molten Solution Download Scientific Diagram

Element Families Of The Periodic Table Informational Text Distance Learning Chemistry Classroom Teaching Chemistry Physical Science Middle School

Pin On Chemistry For A Better Life N Better Society

Nacl Lattice C Magnetic Salt Ions Lattice Salt Crystal Molecule Model
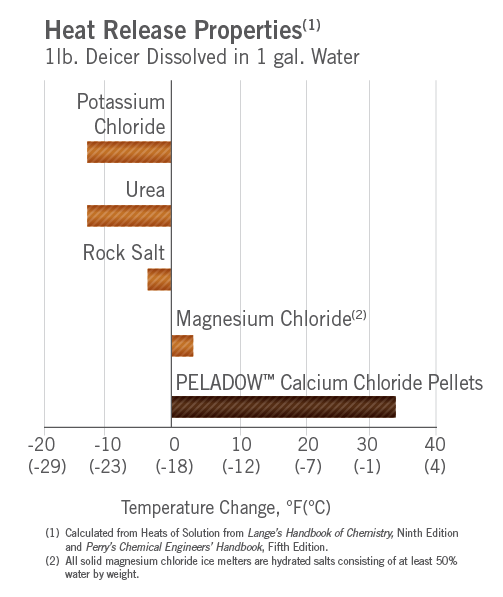
The Case For Calcium Chloride Oxychem Calcium Chloride

What Is Gold Chloride Aucl Powder Molar Mass Chemical Formula Inorganic Compound

Isavuconazonium Sulfate Cosmetic Companies Antifungal Pharmaceutical
Why Is The Melting Point Of Kcl Less Than Nacl Quora

Potassium Tetrachloropalladate Ii K2pdcl4 Crystalline Funcmater Molar Mass Inorganic Compound Chemical Formula

Why Is The Melting Point Of Kcl Less Than Nacl Quora

Stannous Chloride Properties Uses Basic Sophomore Year Sophomore
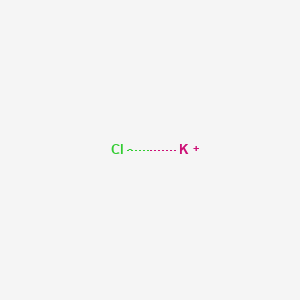
Potassium Chloride Kcl Pubchem

Binary Phase Diagram Of Kcl And Nacl Liq Is A Molten Solution Download Scientific Diagram

Ice Melt Is Ice Melt Here S How To Use It Ice Melting Snow Melting Types Of Ice
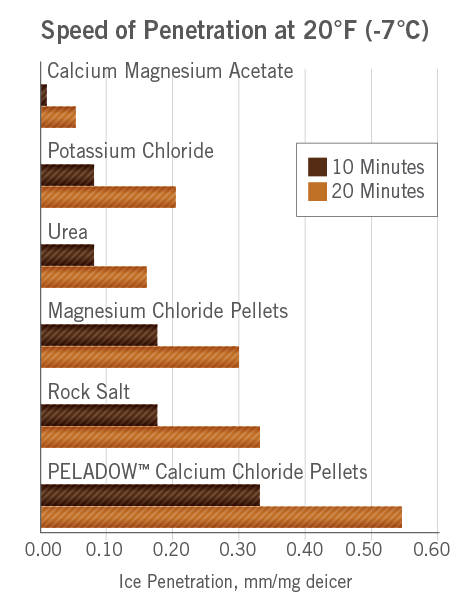
The Case For Calcium Chloride Oxychem Calcium Chloride

Niobium Chloride Nbcl5 Powder Funcmater Chemical Formula Sol Gel Niobium

Comments
Post a Comment